Administering TOFIDENCE
Guide to IV Administration1
Click image for each stage of preparation.
This content is not intended to be medical or professional advice. This is for informational purposes only. Please refer to the full Prescribing Information for dosing and administration instructions and your institution's protocols for IV infusion.
Preparing TOFIDENCE
Visually inspect the solution for particulate matter or discoloration prior to administration. Only solutions which are clear to opalescent, colorless to pale yellow and free of visible particles should be diluted.
TOFIDENCE for intravenous infusion should be diluted by a healthcare professional using aseptic technique. Use a sterile needle and syringe to prepare TOFIDENCE.
Fully diluted TOFIDENCE solutions are compatible with infusion bags and/or infusion sets with the following materials: polypropylene, polyethylene, polyolefin, polyvinyl chloride, polyethersulfone, polyurethane, nylon and stainless steel.
This content is not intended to be medical or professional advice. This is for informational purposes only. Please refer to the full Prescribing Information for dosing and administration instructions and your institution's protocols for IV infusion.
TOFIDENCE for intravenous infusion should be diluted by a healthcare professional using aseptic technique as follows:
- Use a sterile needle and syringe to prepare TOFIDENCE.
- Patients weighing less than 30 kg: use a 50 mL infusion bag or bottle of 0.9% Sodium Chloride Injection, USP, and then follow steps 1 and 2 below.
- Patients weighing 30 kg weight or above: use a 100 mL infusion bag or bottle, and then follow steps 1 and 2 below.
Step 1
Withdraw a volume of 0.9% Sodium Chloride Injection, USP, equal to the volume of the TOFIDENCE injection required for the patient’s dose from the infusion bag or bottle.
For Intravenous Use: Volume of TOFIDENCE Injection per kg of Body Weight1
| Dosage | Indication | Volume of TOFIDENCE injection per kg of body weight |
|---|---|---|
| 4 mg/kg | Adult RA* | 0.2 mL/kg |
| 6 mg/kg | Adult GCA | 0.3 mL/kg |
| 8 mg/kg | Adult RA Adult COVID-19 PJIA and SJIA (≥30 kg of body weight) |
0.4 mL/kg |
| 10 mg/kg | PJIA (<30 kg of body weight) |
0.5 mL/kg |
| 12 mg/kg | SJIA (<30 kg of body weight) |
0.6 mL/kg |
*For adult patients with RA, when used in combination with DMARDs or as monotherapy, the recommended starting dose is 4 mg per kg every 4 weeks followed by an increase to 8 mg per kg every 4 weeks based on clinical response.
Step 2
Withdraw the amount of TOFIDENCE for intravenous infusion from the vial(s) and add slowly into the 0.9% Sodium Chloride Injection, USP infusion bag or bottle. To mix the solution, gently invert the bag to avoid foaming.
The fully diluted TOFIDENCE solutions for infusion using 0.9% Sodium Chloride Injection, USP may be stored refrigerated at 36°F to 46°F (2°C to 8°C) for up to 24 hours or room temperature at 68°F to 77°F (20°C to 25°C) for up to 12 hours and should be protected from light.
TOFIDENCE solutions do not contain preservatives; therefore, unused product remaining in the vials should not be used.
Allow the fully diluted TOFIDENCE solution to reach room temperature prior to infusion.
This content is not intended to be medical or professional advice. This is for informational purposes only. Please refer to the full Prescribing Information for dosing and administration instructions and your institution's protocols for IV infusion.
Step 2 continued
The infusion should be administered over 60 minutes, and must be administered with an infusion set. Do not administer as an intravenous push or bolus.
TOFIDENCE should not be infused concomitantly in the same intravenous line with other drugs. No physical or biochemical compatibility studies have been conducted to evaluate the co-administration of TOFIDENCE with other drugs.
TOFIDENCE for intravenous infusion should be administered over 60 minutes, and must be administered with an infusion set. Do not administer as an intravenous push or bolus.1
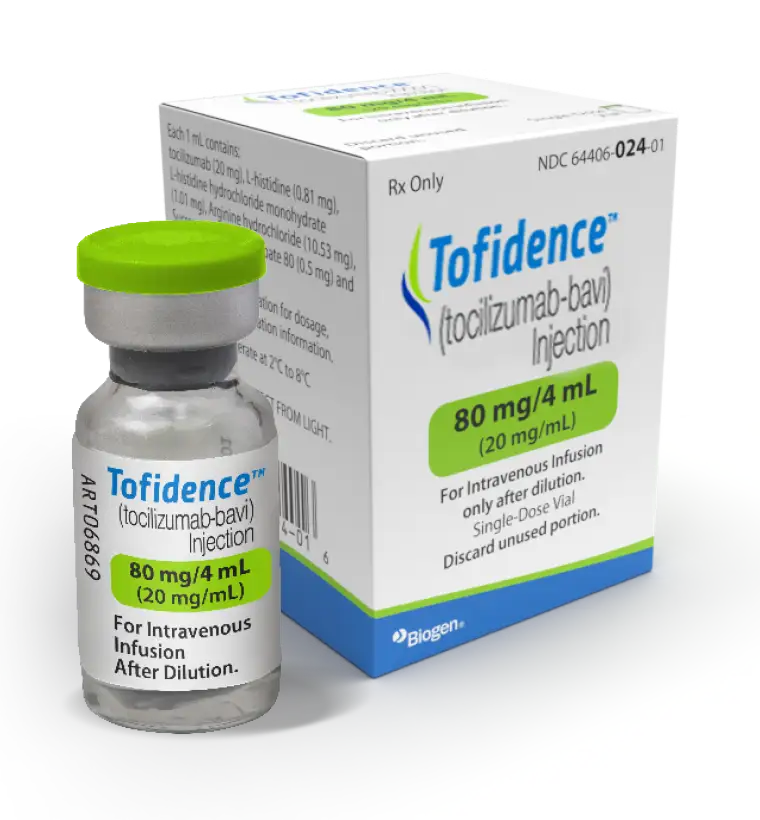
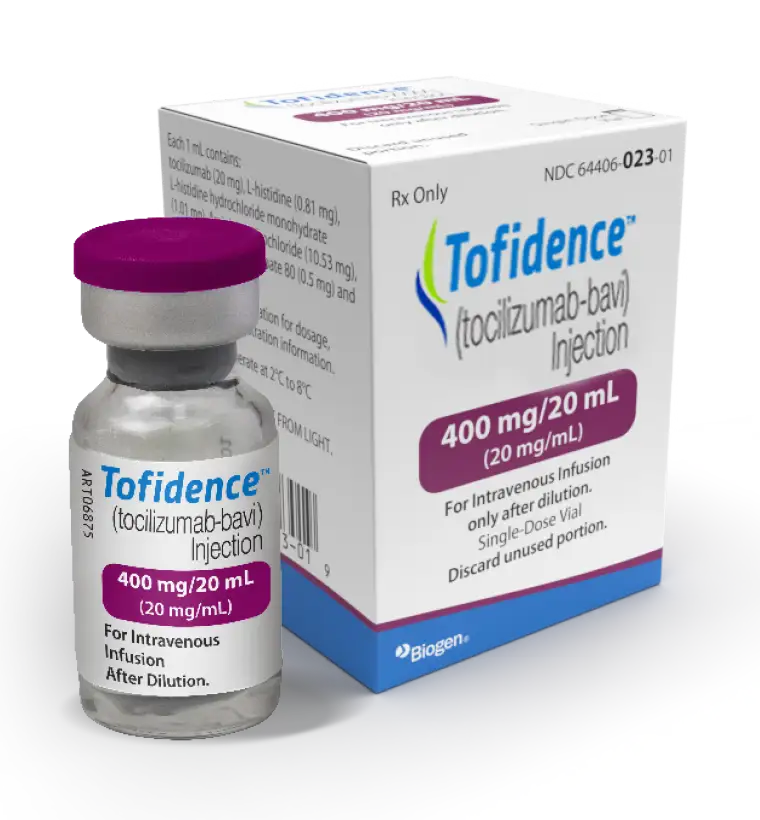
Available in 4, 10, and 20 mL vials at a concentration of 20 mg/mL1*
TOFIDENCE
(tocilizumab-bavi)
80 mg/4 mL


TOFIDENCE
(tocilizumab-bavi)
200 mg/10 mL



TOFIDENCE
(tocilizumab-bavi)
400 mg/20 mL


Not actual size.
*Single-dose vials for further dilution prior to intravenous infusion.
Read the full Prescribing Information for complete dosing and monitoring guidance instructions on how to use TOFIDENCE1
In order to improve the traceability of biological medicinal products, the tradename and batch number of the administered medicinal product should be clearly recorded. Each TOFIDENCE vial contains 80 mg, 200 mg, or 400 mg of tocilizumab-bavi. To calculate the dose and the number of TOFIDENCE vials needed, you can use our dosing calculator and refer to the full Prescribing Information.
Patients treated with TOFIDENCE should be given the medication guide.
Help start a movement for more patients
You also might be interested in:
- TOFIDENCE Prescribing Information, Cambridge, MA: Biogen.
